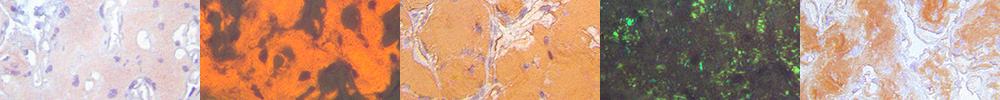
Diagnosing AmyloidosisThe microscopic identification of amyloid and its classification (“typing”) enables the physician to design a therapy which is amyloid class-specific. Therefore, the diagnosis on tissue sections needs to be highly precise and sensitive since ever earlier stages in the amyloidoses are being diagnosed by biopsy today. Early stages are being diagnosed due to the increasing awareness of patients and physicians since modern therapies have been designed and even more improved therapies are arising (1, 2). The advantages of immunohistochemical typing will be addressed in particular.
The diagnosis of amyloid using Congo red
The most commonly applied diagnostic method on tissue sections is the classical Congo red staining method of Puchtler et al. which is also used in our laboratory (3, 4). Diagnostic evaluation is based on the recognition of the affinity for Congo red as seen with normal bright light illumination, as well as the green birefringence seen with plane-polarized light. Diagnosis in our laboratory also involves the use of Congo red fluorescence elicited with short wavelength light, due to several crucial advantages, especially. its higher sensitivity, which improves the classical Congo red method and assists avoiding sampling errors. Fluorescence also avoids the unavoidable “polarisation shadow” effect when evaluating Congo red's green birefringence (4), and it allows proof of the congruence of amyloid and the immunohistochemical overlay (5) for assisting and confirming the correct amyloid class in immunohistochemical typing of amyloid. (6-10).
The Classification (=Typing) of amyloid and amyloidosis
The chemical nature of the amyloids in tissue sections needs to be identified with respect to therapy with high precision and high sensitivity. We apply the immunohistochemical classification of amyloid using our amY-kit amyloid antibodies (6). The specificity of these antibodies was confirmed on
- a large series of formalin-fixed and paraffin-embedded prototype tissue sections (8)
- by submitted samples with clinical data (8)
- by the successful use of our antibodies by several other laboratories
- by two international comparisons (Ring-studies I and II) in comparison with mass spectrometry in a blinded fashion evaluation (in progress)
According to these favourable results compared with mass spectrometry (reports in progress) we have marketed several sets of antibodies; the amY-kit and amY-kit ext/her/vet.
amY-kit is being used by our laboratory for amyloid typing on formalin-fixed and paraffin-embedded tissue sections submitted to us by patients and physicians for amyloid diagnostics, which includes proving the presence of amyloid and the amyloid classification. It contains 10 vials containing 16 antibodies which cover 98% of the most prominent 8 amyloidoses. The precision was calculated from tissue sections which were submitted to our laboratory for typing of amyloid as demanded by a large number of patients.
amY-kit ext (an extended kit) is available for rare and very rare amyloids. These were classified with the panel in cases where amY-kit did not yield a diagnostic result. The evaluation was performed in a comparative way as illustrated and published in detail elsewhere (8-10).
Advantages of our immunohistochemical typing of amyloid
Our immunohistochemical classification of amyloid can be done microscopically on formalin-fixed paraffin sections mounted on routine glass slides . It is precise, easy, fast and inexpensive. It enables the physician to get the correct amyloid type in a day, if needed in his hospital close to the patients’ living area during the early diagnostic phase. Other decisive advantages came to light in two international blinded comparisons with mass spectrometry (in progress).
Due to its higher sensitivity, immunohistochemical typing can classify very early (and even preclinical) amyloid deposits. This would be the ideal stage for diagnosing amyloid before an amyloidosis characterized by organ damage has developed.
References
- Skinner M, Berk JL, Conners, LH, Seldin, DC. XIth International Symposium on Amyloidosis. CRC Press, Taylor&Francis Group, pp. 1-406; Boca Raton, London, New York 2006.
- Merlini, G. XIIth International Symposium on Amyloidosis. Amyloid 16, Suppl 1, pp 1 -237 (2011).
- Linke, RP: Highly sensitive diagnosis of amyloid and various amyloid syndromes using Congo red fluorescence. Virchows Arch. Path. Anat. 436:439-448 (2000).
- Linke, RP: Congo red staining of amyloid. Improvements and practical guide for a more precise diagnosis of amyloid and the different amyloidoses. In: “Protein misfolding, aggregation and conformational diseases” (VN Uversky and AL Fink, eds.), Protein Reviews, Volume 4, (MZ Atassi, ed.); Chapter 11.1, pp. 239-276; 2006 Springer.
- Linke, R.P., Gärtner, V., Michels, H.: High sensitivity-diagnosis of AA amyloidosis using Congo red and immunohistochemistry detects missed amyloid deposits. J. Histochem. Cytochem. 43, 863-869 (1995).
- Schröder R., Deckert M., Linke R.P.: Novel isolated cerebral ALlambda amyloid
angiopathy with widespread subcortical distribution and leukoencephalopathy
due to atypical monoclonal plasma cell proliferation, and terminal systemic
gammopathy. J Neuropathol Exp Neurol. 68: 286-299 (2009).
- Linke, R.P., Oos, R., Wiegel, N.M., and Nathrath, W.B.J (2006): Classification of amyloidosis: misdiagnosing by way of incomplete immunohistochemistry and how to prevent it. Acta Histochem 108, 197-208.
- Linke, R.P.: Classification of amyloid on fixed tissue sections for routine use by validated immunohistochemistry. Amyloid 18, Supp. 1, 74-76 (2011).
- Linke R.P.: Diagnosis of minimal amyloid deposits using the Congo red fluorescent method: a review. In: Amyloid and Related Disorders: Surgical Pathology and Clinical Correlations; Current Clinical Pathology. Picken MM, et al (eds.) Chapter 13, pp 175-185; Springer Science & Business Media. 2012.
- Linke, R.P.: Routine use of amyloid typing on formalin-fixed paraffin sections from 626 patients by immunohistochemistry. In: Amyloid and Related Disorders: Surgical Pathology and Clinical Correlations, Current Clinical Pathology. Picken MM, et al, (eds.) Chapter 17, pp 219-229; Springer Science & Business Media. 2012.
- Linke R.P.: On typing amyloidosis using immunohistochemistry. Detailed illustrations, review and a note on mass spectrometry. Prog. Histochem. Cytochem. 47: 61-132 (2012).
- Linke R.P.: Amyloidosen. In “Klinische Immunologie” HH Peter, WJ Pichler und U Müller-Ladner (Hrsg); 3. Auflage, Kapitel 17.1, 725-738; Urban & Fischer München 2012.
|